Video misleads about safety of 6-in-1 vaccine Vaxelis
- Published on June 10, 2025 at 19:04
- 5 min read
- By Marisha GOLDHAMER, AFP Canada, AFP USA
"A new vaccine is on the market for your 6 week old baby! It's never been tested against a placebo or for carcinogenic effects," claims a May 19, 2025 Instagram post from a Canadian content creator who regularly posts against vaccination.
She is one of several creators who stitched their reactions next to a video with thousands of interactions from Jeffrey Barke, a founding member of America's Frontline Doctors -- a group AFP has repeatedly fact-checked.
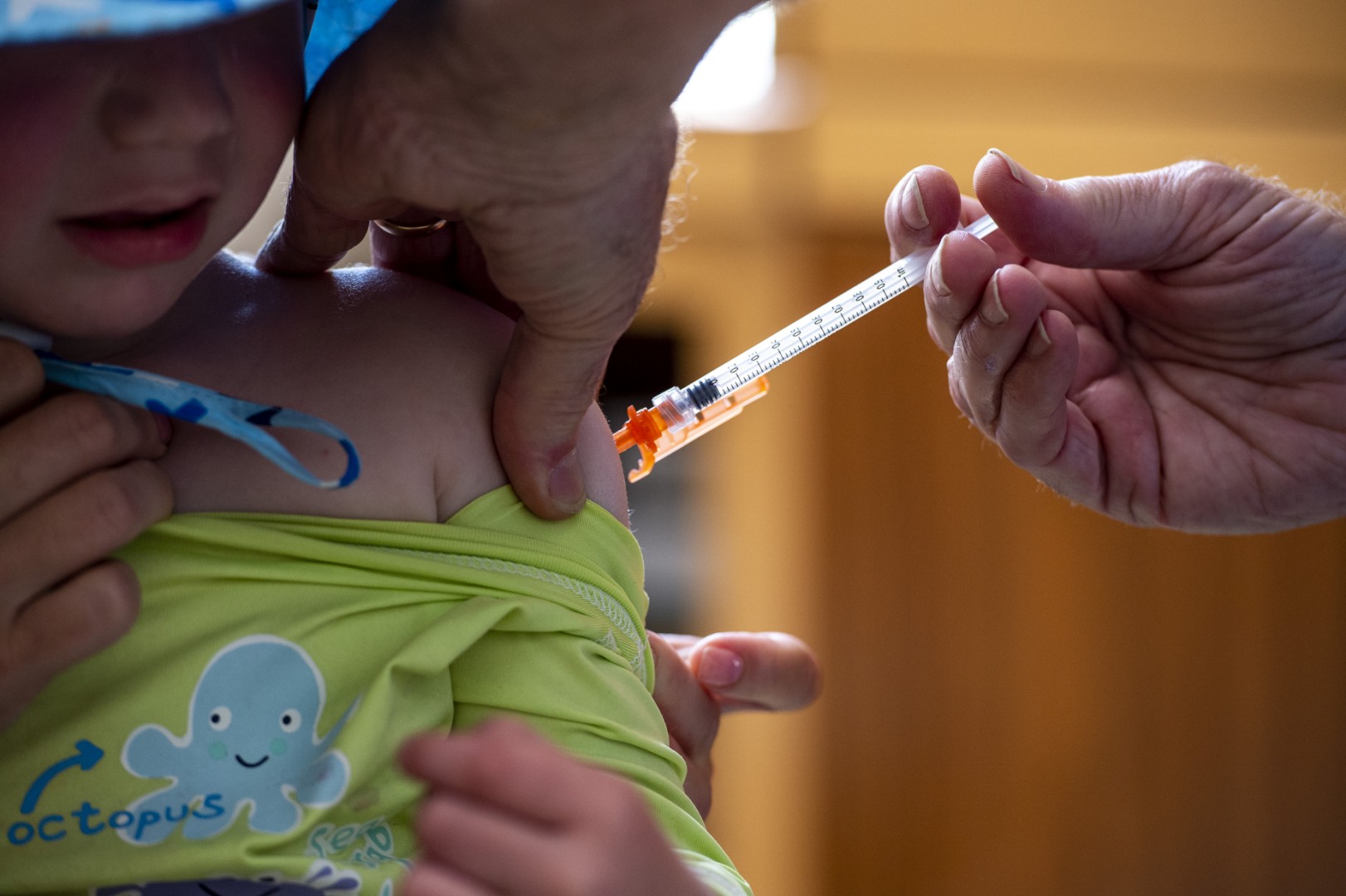
In the video, Barke reads from the package insert for Vaxelis, a shot given to simultaneously prevent infections caused by pertussis, diphtheria, tetanus, poliomyelitis, Haemophilus influenzae type b, and hepatitis B (archived here).
"Studies that brought this product to market -- there is not a single randomized controlled study with an inert placebo. It's only tested against other vaccinations," he says, implying this is cause for concern.

Similar claims also appeared on X in French, German, Spanish and Croatian, spreading as officials with the US Department of Health and Human Services under Robert F. Kennedy, Jr. aim to shift the way vaccines are tested and approved.
The posts also come as experts say vaccine misinformation is rampant online and may be contributing to falling vaccination rates across the United States and Canada (archived here and here).
Vaxelis is not new, however. The US Food and Drug Administration first approved the joint venture of pharmaceutical companies Sanofi Pasteur and Merck for children six weeks through four years of age in December 2018 (archived here and here). It is also administered in Europe (archived here).
Kelly Moore, president and CEO of the nonprofit Immunize.org, co-authored the policy statement for Vaxelis from the US Advisory Committee on Immunization Practices (ACIP), a panel of experts that makes recommendations about the childhood vaccinations schedule (archived here).
"It's an excellent vaccine," she told AFP in a June 2 email.
In February 2019, the ACIP reported that Vaxelis was subjected to six clinical studies involving more than 5,000 infants six to 12 weeks of age (archived here). It said the studies showed Vaxelis "had an acceptable safety profile that is consistent with its component vaccines."
Placebo testing
Barke is correct that Vaxelis was not tested against an inert placebo. But experts said there is reason for that: it would have been unethical to do so.
Clinical trial participants must be offered the existing standard of care, Moore said. It would be "entirely unethical" to withhold existing vaccines while testing a combination product that contains components already routinely given in those separate shots, she said.
The World Health Organization has also pushed back against calls for changes to vaccination testing.
"The safety of vaccines is held to an extremely high standard," WHO vaccine chief Kate O'Brien said May 1, 2025 (archived here).
O'Brien stressed that the "gold standard" process calls for placebo testing when developing vaccines against diseases for which no immunization options exist.
But when vaccines are developed to cover new strains or to offer a combination shot against multiple viruses, they are generally tested to see if they are as or more effective than the existing shots.
O'Brien said it would also be unethical to give test subjects a placebo in place of "vaccines that are life-saving that are already licensed."
Combination vaccines
Barke further misleads by misinterpreting section 13.1 of the package insert, which says that Vaxelis was not evaluated for "carcinogenic or mutagenic potential or impairment of fertility."
The language appears in documents for many routine vaccines, US health care group Novant Health explains on its website (archived here).
"This merely means that there was no need for further testing because toxicology studies conducted at the preclinical phase showed no signs of adverse effects from the vaccine or its individual components," it says.
There are also specific guidelines and rules for when a manufacturer needs to perform fertility studies.
With a shot approved for use only in populations under five years old, studies in pregnant animals would not take place, said Kathryn Edwards, an expert in vaccinology at the Vanderbilt University Medical Center (archived here).
"These vaccines have been used individually for decades in millions of people that there has not been a signal for infertility and no biologic reason why you would be concerned," she said in a June 4 email.
Six-in-one shots, including Vaxelis, were developed to lower the number of injections for children, "reduce the burden of handling multiple different separate vaccines and to ensure timely administration of all the needed vaccines," Edwards said.
The Canadian Paediatric Society and the Canadian National Advisory Committee on Immunization currently recommend children receive 5-in-1 or 6-in-1 vaccines (archived here and here).
After a vaccine is approved, its safety continues to be monitored.
"Combination vaccines have been shown to be no more reactive than separate vaccines, and no long term complications have been seen with combination vaccines," Edwards said.
Such products have several advantages, including increased coverage rates, reduced need for additional health care visits and lower costs for distributing the product, according to the US Center for Disease Control and Prevention (archived here).
Adverse effects
Barke's video highlights the potential risk of Guillain-Barré syndrome -- a condition in which the body's immune system attacks the nerves, sometimes causing temporary paralysis. He also points to apnea, which is a pause in breathing, as a risk.
But Edwards said Guillain-Barré syndrome is "very rare in children."
Cedars Sinai Hospital says on its website that most children diagnosed with Guillain-Barré syndrome "recover fully with no complications" (archived here).
Rachel Greenberg, associate professor of pediatrics at Duke University School of Medicine, researched apnea risks when vaccinating premature infants (archived here). "While there is a temporary increased risk of apnea after vaccination, the risk posed by vaccine-preventable respiratory and other infections to unvaccinated infants is far higher," she said in a press release about her January 2025 study (archived here).
The most common side effects reported following a shot of Vaxelis are pain or swelling at the injection site and fever (archived here).
Melody Butler, founding executive director of Nurses Who Vaccinate, said parents weighing the risk of rare, serious adverse events should remember how many children still die from vaccine-preventable diseases (archived here).
"We've become so spoiled that we don't even know what these diseases do to the human body," she told AFP June 4. "We're very fortunate that we don't see these diseases firsthand."
Ingredients
Barke's video also targets the vaccine's ingredients, a tactic regularly deployed by activists who claim, without evidence, that immunizations are dangerous.
In particular, he questions aluminum and formaldehyde.
Aluminum is naturally found in the environment, and humans ingest it through drinking water and some foods, including breast milk and infant formula.
Aluminum boosts immune response, reducing the amount of vaccine required to provide immunity (archived here).
Aluminum-containing vaccines have been used for decades and have been administered to more than one billion people without problem, the Children's Hospital of Philadelphia (CHOP) says on its website (archived here).
Formaldehyde, meanwhile, is "essential in human metabolism" and can be found in all humans, according to CHOP (archived here).
It is used in vaccines to help prevent bacterial contamination during manufacturing (archived here).
"There's more formaldehyde in a single apple or pear than in a vaccine," Butler said, dismissing the residual amounts passed to the body during vaccination as "negligible."
Read more of AFP's reporting on vaccine misinformation here.
Copyright © AFP 2017-2026. Any commercial use of this content requires a subscription. Click here to find out more.
Is there content that you would like AFP to fact-check? Get in touch.
Contact us