Misleading claims on US flu shot ingredients recirculate
- This article is more than two years old.
- Published on December 26, 2023 at 16:54
- Updated on December 26, 2023 at 18:03
- 4 min read
- By Marisha GOLDHAMER, AFP USA
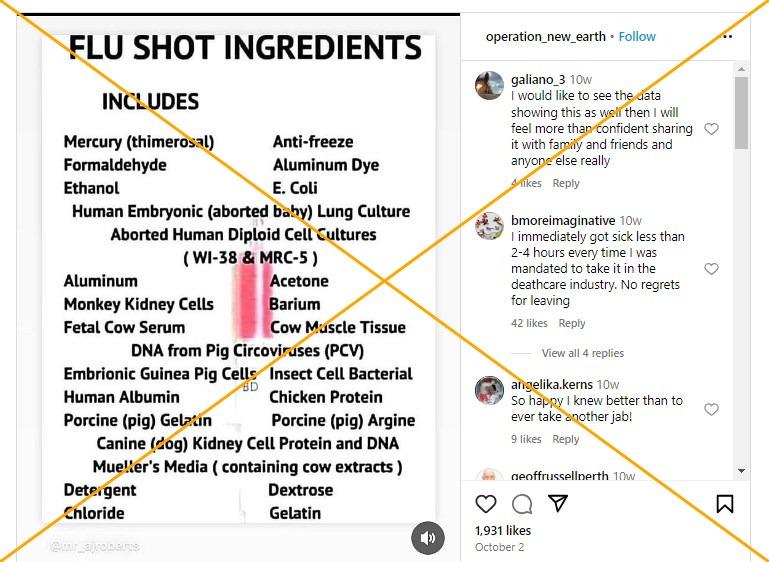
"Flu shot ingredients," is the title of a graphic shared 1,400 times on X, formerly Twitter, since December 5, 2023. The image which claims the shots include substances such as mercury and acetone, spread in thousands of posts on social media and websites as public health campaigns ramped up to encourage annual inoculation.

Misinformation about the flu shot is an annual occurrence and experts have previously told AFP that the ingredients used in vaccines available in the United States and Canada have proven safety records.
The US Centers for Disease Control and Prevention recommends the jab for almost everyone six months and older (archived here). From October 1 to December 16, the CDC estimates at least 3,200 deaths and 54,000 hospitalizations were caused by influenza in the United States (archived here).
Each year the vaccine is reformulated in an effort to match the strains of flu in circulation, based on recommendations from the World Health Organization and experts say this year's formulation is very protective against severe illness.
In the United States, nine vaccines are approved for use in the 2023/24 flu season from the companies Seqirus, GlaxoSmithKline, Sanofi Pasteur and AstraZeneca.
Afluria Quadrivalent, Fluarix Quadrivalent, FluLaval Quadrivalent, Fluzone Quadrivalent and Flucelvax Quadrivalent are available to patients age six months and older (archived here, here, here, here and here). Flublok Quadrivalent is only for people older than age 18 (archived here). While Fluzone High-Dose Quadrivalent and Fluad Quadrivalent are for those aged 65 and up (archived here and here). The nasal spray vaccine FluMist Quadrivalent is available to those aged 2-49 (archived here).
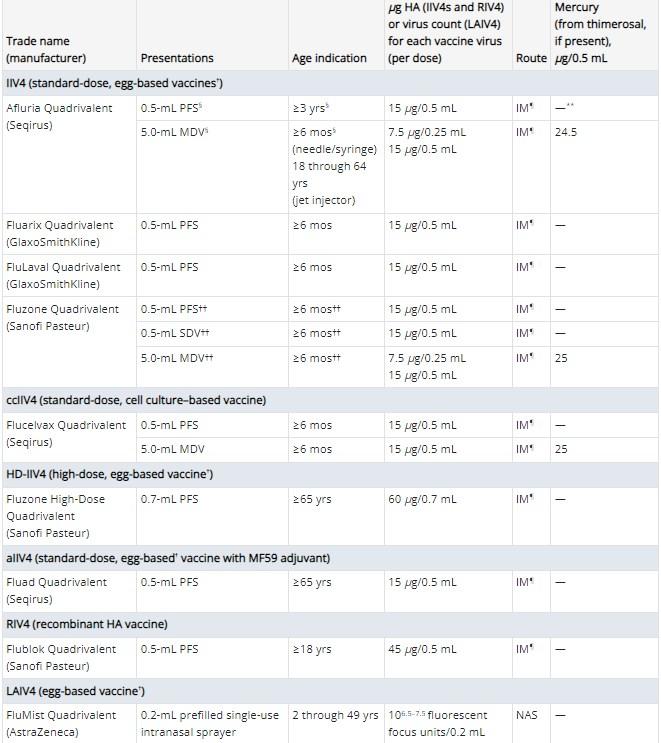
AFP examined the active and inactive ingredients listed for the available flu shots and compared them to the list in the viral image. Most of those in the claim are not included, with thimerosal, formaldehyde and gelatin found in some of this year's jabs.
The CDC website says all vaccine ingredients are selected to make the shot "as effective as possible while being safe." They can be grouped by their different functions as explained below:
Preservatives
To prevent contamination, vaccines may include preservatives such as thimerosal.
Thimerosal contains ethylmercury, which is different from methylmercury, a toxic organic compound.
"Ethylmercury is safe to use in vaccines because it's processed differently in the body and is less likely to build up in the body -- and because it's used in tiny amounts," the CDC says on its website (archived here).
"Only multi-dose vial formulations of influenza vaccines will contain thimerosal," according to the CDC (archived here), so patients can request a shot that does not contain the preservative if they wish.
Additionally, Fluarix Quadrivalent, Flublok Quadrivalent, Fluzone High-Dose Quadrivalent, Fluad Quadrivalent and FluMist Quadrivalent are formulated without preservatives.
Residual inactivating ingredients
Some ingredients help kill viruses or inactivate toxins during the manufacturing process. Formaldehyde is sometimes used in this way.
"The amount of formaldehyde present in some vaccines is so small compared to the concentration that occurs naturally in the body that it does not pose a safety concern," the US Food and Drug Administration says on its website (archived here).
Five of the shots include formaldehyde.
Residual cell culture materials
To grow enough of the virus or bacteria to make the vaccine, there may be residual cells in the formula. Most of the flu shots in the US are egg-based and may include proteins from the process. This may account for why the image shared on social media mentions "chicken protein."
The CDC recommends the vaccines, even if a person has a known egg allergy.
"Egg allergy does not indicate additional safety measures for flu vaccination beyond those recommended for any recipient of any vaccine, regardless of severity of previous reaction to egg," it says online (archived here).
Adjuvants
The purpose of including certain adjuvant ingredients in a vaccine is to increase immune response, Ashlesha Kaushik, an expert in pediatric infectious disease, told AFP for a previous fact-check.
"Adjuvants make vaccines more effective," she said.
None of this year's vaccines include aluminum, but it is used in other vaccines and has a six-decade safety profile.
Stabilizers
To keep the vaccine effective after manufacturing, stabilizing ingredients are added.
Gelatin is derived from pigs and is used as a stabilizer in two of this year's flu vaccines, including FluMist Quadrivalent.
The University of Oxford's Vaccine Knowledge website explains that the gelatin in vaccines is highly purified and testing has shown that "no DNA from pigs can be detected in the nasal flu vaccine" (archived here).
AFP has previously debunked multiple claims about vaccines, including detailed looks at the safety profile of the ingredients potassium chloride and polysorbate 80.
December 26, 2023 The caption of the lead photo was updated.
Copyright © AFP 2017-2026. Any commercial use of this content requires a subscription. Click here to find out more.
Is there content that you would like AFP to fact-check? Get in touch.
Contact us