Social media posts misleadingly describe lenacapavir as a vaccine
- Published on August 8, 2025 at 17:53
- 3 min read
- By Peris GACHAHI, AFP Kenya
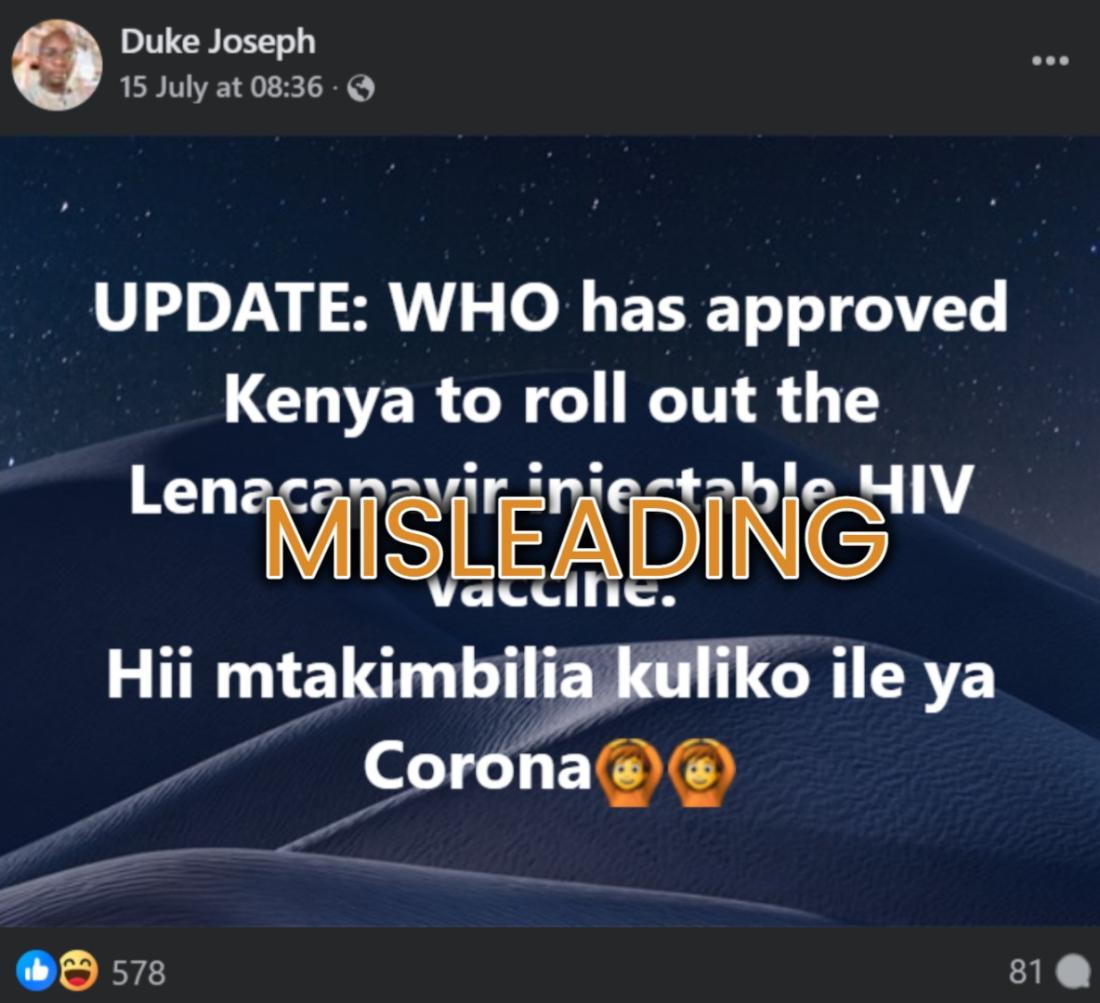
“UPDATE: WHO has approved Kenya to roll out the Lenacapavir injectable HIV vaccine. You will rush for this more than the one for Corona,” reads an English and Swahili Facebook post published on July 15, 2025.
Similar claims were published here and here on Facebook.
HIV breakthrough
HIV is a virus that attacks the body’s immune system by targeting white blood cells. These cells are essential in helping the body fight against infections and diseases (archived here).
It is transmitted through contact with bodily fluids such as blood and semen from an infected person, and if left untreated, HIV can advance to AIDS (Acquired Immunodeficiency Syndrome), the most severe stage of infection.
At the end of 2024, the World Health Organization (WHO) estimated that 40.8 million people were living with HIV globally, with 65 percent of them in the WHO African Region.
Administered as a twice-yearly injection and developed by Gilead Sciences, LEN represents a major advancement in the fight against HIV and was approved by the US Food and Drug Administration on July 1, 2025 (archived here and here).

During the 13th International AIDS Society Conference (IAS 2025) on HIV Science in Kigali, Rwanda, this July, the WHO also endorsed the drug, issuing guidelines recommending it as an additional pre-exposure prophylaxis (PrEP) option for people at high risk of HIV infection (archived here).
PrEP is medication taken by individuals who do not have HIV but are at risk of contracting it. In case of infection, these treatments help the body stop the virus from replicating (archived here).
Kenya was selected as one of the nine early adopter countries for the rollout of LEN, scheduled for January 2026. According to the country’s health ministry, each dose is expected to cost 6,000 Kenyan shillings (about $46) (archived here and here).
However, social media posts describing LEN as a vaccine are misleading.
Not a vaccine
According to its manufacturer, Gilead Sciences, LEN, marketed under the brand names Sunlenca and Yeztugo, is approved for two uses: as a treatment for adults who are resistant to other HIV treatments -- combined with other ARVs -- and as a PrEP option to reduce the risk of sexually acquired HIV (archived here and here).
ARVs are used to treat HIV. They do not cure the virus but are a lifelong treatment that works by suppressing the infection to prevent it from progressing to AIDS (archived here).
Kenya’s director general for health, Dr. Patrick Amoth, told AFP Fact Check: “Lenacapavir is not a vaccine. It is an antiretroviral medicine that prevents HIV infection.”
This is different from how a vaccine affects the body.
Vaccines train the immune system to recognise and fight specific pathogens before a person is infected. They do so by imitating an infection to trigger the body’s natural defences. This helps the body identify the pathogen so it can respond quickly if exposed to it in the future, preventing disease (archived here).
In contrast, ARVs like LEN are used to treat those already living with HIV or to prevent infection in the case of PrEP. These drugs work by blocking the virus at different stages of its lifecycle, and when taken consistently, they help control the infection and prevent transmission to others (archived here).
“Vaccines work by stimulating the body to produce antibodies against a disease,” Amoth said. “Lenacapavir is a capsid inhibitor, blocking HIV from replicating by interfering with viral assembly and release, hence allowing the body to prevent new infections.”
The capsid is the protein shell of an infectious virus that encases its genetic material. Capsids help viruses such as HIV survive. Drugs that are capsid inhibitors, like LEN, disrupt viral replication and protect the host from infection (archived here and here).
Dr Andrew Mulwa, head of Kenya’s National AIDS & STI Control Program (NASCOP), reiterated that LEN is not a vaccine.
“Lenacapavir, just like Cabotegravir, is an antiretroviral drug,” Mulwa told AFP Fact Check.
Cabotegravir, marketed as Apretude and Cabenuva, was the first long-acting injectable PrEP that was approved by the FDA in 2021. It is administered as two initial injections four weeks apart, followed by an injection every two months (archived here).
During his opening remarks at the IAS 2025 conference, WHO Director-General Dr Tedros Adhanom Ghebreyesus said: “While an HIV vaccine remains elusive, lenacapavir is the next best thing: a long-acting antiretroviral shown in trials to prevent almost all HIV infections among those at risk” (archived here).
AFP Fact Check has debunked other HIV-related claims here.
Copyright © AFP 2017-2026. Any commercial use of this content requires a subscription. Click here to find out more.
Is there content that you would like AFP to fact-check? Get in touch.
Contact us