False claims resurface on RSV infant immunization
- This article is more than one year old.
- Published on July 16, 2024 at 18:33
- 3 min read
- By Daniel FUNKE, AFP USA
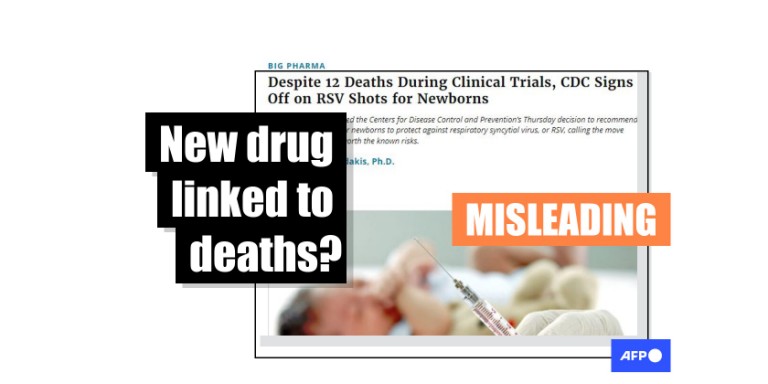
"Despite 12 Deaths During Clinical Trials, CDC Signs Off on RSV Shots for Newborns," says an August 4, 2023 headline from Children's Health Defense, an organization that AFP has repeatedly fact-checked for spreading vaccine misinformation.
DiedSuddenly, an X account whose name references a debunked film about the supposed dangers of Covid-19 vaccines, resurfaced the article in a July 9, 2024 post with tens of thousands of interactions.
"Murderers," the screenshot's caption says.

Similar claims spread elsewhere on X and Facebook, including in Canada.
RSV is "a common respiratory virus that usually causes mild, cold-like symptoms," according to the US Centers for Disease Control and Prevention (CDC). However, infants and older adults are more likely to develop severe cases requiring hospitalization (archived here).
RSV outbreaks in recent years have resulted in a surge of misinformation about the virus, including unproven remedies and debunked claims about vaccines.
The latest narrative is an outgrowth of that trend.
What is nirsevimab?
Nirsevimab, sold under the brand name Beyfortus, is a monoclonal antibody drug that the FDA approved in July 2023 to prevent RSV and related complications in young children (archived here). Regulators had previously authorized the drug in the European Union, United Kingdom and Canada (archived here, here and here).
US clinical trials found one injection of nirsevimab was more than 70 percent effective at reducing a patient's risk of receiving medical treatment for the virus. The drug works by introducing lab-made proteins that "mimic the immune system's ability to fight off harmful pathogens such as viruses," the FDA says on its website.
Monoclonal antibodies differ from vaccines in that they do not teach the body how to fend off future infections, making them more useful for providing immediate and short-term protection from conditions such as cancer and Covid-19 (archived here, here, here and here).
The CDC recommends "either maternal RSV vaccination or infant immunization with RSV monoclonal antibodies," adding on its website that "most infants will not need both" (archived here). The American Academy of Pediatrics also recommends nirsevimab for preventing RSV in children (archived here).
The CDC says temporary pain, redness and swelling at the injection site are potential side effects of preventive antibodies (archived here). Nirsevimab is not recommended for children with a history of severe allergic reactions and the agency says it should be "given with caution" to those with bleeding disorders (archived here).
'Important' countermeasure
An FDA briefing document indicates 11 deaths were reported among infants who received the shot during clinical trials, while one more died after receiving another RSV drug called palivizumab (archived here).
However, the agency told AFP there is no connection to the injections, as the social media posts claim.
"The FDA carefully reviewed the cases of deaths reported in pivotal nirsevimab studies through 360 days post-dose to assess causality, and concluded that none of the deaths in the clinical trials were considered to be related to Beyfortus," a spokesperson said in a July 11, 2024 statement.
"The recorded deaths were attributed to underlying conditions such as congenital heart disease or other causes of infant mortality in the respective country or region where the trial subject was enrolled, such as untreated gastroenteritis."
A review of Beyfortus from the Center for Drug Evaluation and Research detailed the causes for each death reported in clinical trials (archived here). One child in Panama suffered a skull fracture in a car accident, while a premature infant in Ukraine died from Covid-19.
"Individuals in clinical trials may die from unrelated causes, and these deaths were subject to discussion and investigation and did not reveal a link with the treatment," said Amesh Adalja, a senior scholar at the Johns Hopkins Center for Health Security (archived here), in a July 11 email.
"Nirsevimab is an important RSV medical countermeasure and ... was just shown to be highly efficacious at preventing hospitalization of infants."
AFP previously fact-checked claims in French that Beyfortus is "toxic" for infants.
Copyright © AFP 2017-2026. Any commercial use of this content requires a subscription. Click here to find out more.
Is there content that you would like AFP to fact-check? Get in touch.
Contact us