Posts misleadingly link Pfizer's Covid-19 vaccine to blood clots
- This article is more than three years old.
- Published on December 23, 2022 at 18:57
- 3 min read
- By Daniel FUNKE, AFP USA
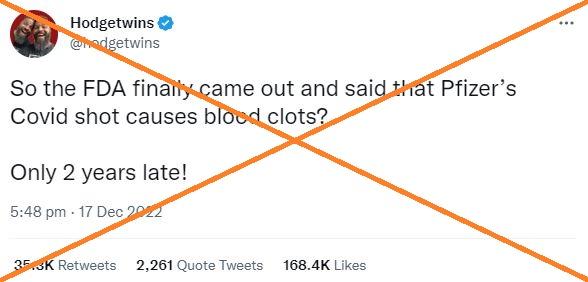
"So the FDA finally came out and said that Pfizer's Covid shot causes blood clots? Only 2 years late!" says a December 17, 2022 tweet from the Hodgetwins, a pair of conservative political commentators in the US.
The post accumulated more than 35,000 shares and drew the attention of Twitter owner Elon Musk, who replied: "Much will come to light as (Anthony) Fauci loses power."
The assertion, which spread amid a winter surge of Covid-19 cases, has also circulated among conservative influencers on Facebook and Instagram -- as well as on websites such as the Republic Brief and the Gateway Pundit, which AFP has fact-checked numerous times.
"After two years, FDA finally admitted Pfizer's Covid-19 vaccine had been linked to blood clotting in older individuals based on the result of one of the largest studies of elderly persons aged 65 years and above," the Gateway Pundit said in its December 18 article.
The claims come on the heels of a debunked film that featured embalmers connecting the Covid-19 vaccines to blood clots. The more recent allegations are based on a study co-authored by researchers affiliated with the FDA, but the social media posts misrepresent their findings.
The study, titled "Surveillance of Covid-19 vaccine safety among elderly persons aged 65 years and older" and published December 1 in the peer-reviewed journal Vaccine, analyzed data from the US Centers for Medicare and Medicaid Services (CMS). The researchers "evaluated 14 outcomes of interest following Covid-19 vaccination" among "30,712,101 elderly persons."
According to the study, four of those outcomes -- including pulmonary embolism, a blood clot that blocks blood flow in the lungs -- "met the threshold for a statistical signal" following vaccination with Pfizer's shot, BNT162b2. But that does not mean the vaccine causes blood clots.
"Our new findings of statistical signals for four important outcomes for the BNT162b2 vaccine should be interpreted cautiously because the early warning system does not prove that vaccines cause the safety outcomes," the study says.
The authors noted several limitations, including the fact that the analysis "did not adjust for underlying risk factors" that could have led to "falsely positive or negative signals." The results also "may not be generalizable to those younger than 65 years and adults who are uninsured or received only commercial health insurance," the study says.
"Because an early warning system does not prove that the vaccines cause these outcomes, more robust epidemiologic studies with adjustment for confounding, including age and nursing home residency, are underway to further evaluate these signals," the paper adds.
AFP reached out to the corresponding study author for additional comment, but a response was not forthcoming.
FDA recommends Pfizer vaccine
Researchers affiliated with the FDA, CMS and private entities conducted the study cited in the social media posts. But the FDA still recommends Pfizer's Covid-19 vaccine for children and adults.
"The FDA continues to find that the Pfizer-BioNTech Covid-19 Vaccine meets the FDA's rigorous standards for safety and effectiveness and the agency strongly believes the potential benefits of Covid-19 vaccination outweigh the potential risks of Covid-19," said Abby Capobianco, an agency press officer, in a statement emailed to AFP on December 21.
She added: "The FDA has not found any new causal relationships between the Pfizer-BioNTech Covid-19 Vaccine and potential adverse events of special interest identified in 2021."
The study notes the FDA is "currently not taking any regulatory actions based on these signal detection activities because these signals are still under investigation and require more robust study." In an online statement updated December 22, the agency said it plans to release additional findings in "early 2023."
A Pfizer spokesperson told AFP the company is cooperating with the FDA and other regulatory authorities as they "independently monitor the safety profile of our vaccine."
"The Pfizer-BioNTech Covid-19 vaccine has been administered to hundreds of millions of adults, adolescents and children generating robust data demonstrating a favorable safety profile and high level of protection against severe Covid-19 disease, including death and hospitalization," the spokesperson said in a statement emailed December 20.
The US Centers for Disease Control and Prevention (CDC) is monitoring some rare "adverse events of interest" reported after Covid-19 vaccination -- including thrombosis with thrombocytopenia syndrome, which causes blood clotting. However, the condition has occurred in approximately four cases per one million doses administered of Johnson and Johnson's Covid-19 vaccine, not Pfizer's.
The CDC says on its website that "severe reactions after vaccination are rare."
More of AFP's reporting on vaccine misinformation can be found here.
Copyright © AFP 2017-2026. Any commercial use of this content requires a subscription. Click here to find out more.
Is there content that you would like AFP to fact-check? Get in touch.
Contact us