Social media posts mislead on South Korean coronavirus vaccine
- This article is more than five years old.
- Published on January 15, 2021 at 11:21
- 2 min read
- By Richard KANG, AFP South Korea
The claim was shared here on Facebook on January 12, 2021.

The Korean text in the post translates to English as: “I called the Ministry of Health and Welfare ten times to ask what type of free vaccines will be given to people. I made calls to them repeatedly, and they finally answered with honesty.
"(They said free vaccines are) not from Pfizer, Moderna or AstraZeneca.
"The free ones will be from Celltrion."
The claim was shared alongside a screenshot of a live broadcast of the South Korean President Moon Jae-in on January 11, 2021, giving a New Year’s Address.
At that time, Moon said: “Together with the people, the government will do everything possible to put an end to the third wave of Covid-19 infections at the earliest date possible.
“Vaccinations can start next month. We will see to it that all citizens will be inoculated free of charge, starting with those prioritised.”
Similar claims have been shared on Facebook here, here and here and on Twitter here.
However, the claim is false.
During the address, President Moon did not mention what kind of coronavirus vaccines would be offered to South Koreans.
Jeong Jae-min, a spokesperson at the Ministry of Health and Welfare, told AFP during a phone interview on January 13, 2021, that the Korea Disease Control and Prevention Agency (KDCA) is in charge of dealing with inquiries regarding Covid-19.
KDCA spokesperson Choi Seung-ho told AFP during a call on January 13, 2021 that Celltrion is currently developing Covid-19 treatment, not a vaccine.
Kim Tae-kyun, a spokesperson at Celltrion, told AFP on the same day that the company is not developing or producing Covid-19 vaccines and has no plan to do so.
The South Korean pharmaceutical giant announced in a statement on January 13, 2021 that the clinical data on the second-phase trial study of the CT-P59, its anti-Covid-19-monoclonal antibody treatment candidate, reduced the rate of Covid-19 patients developing severe cases by 54 percent, while it cut recovery time more than three days.
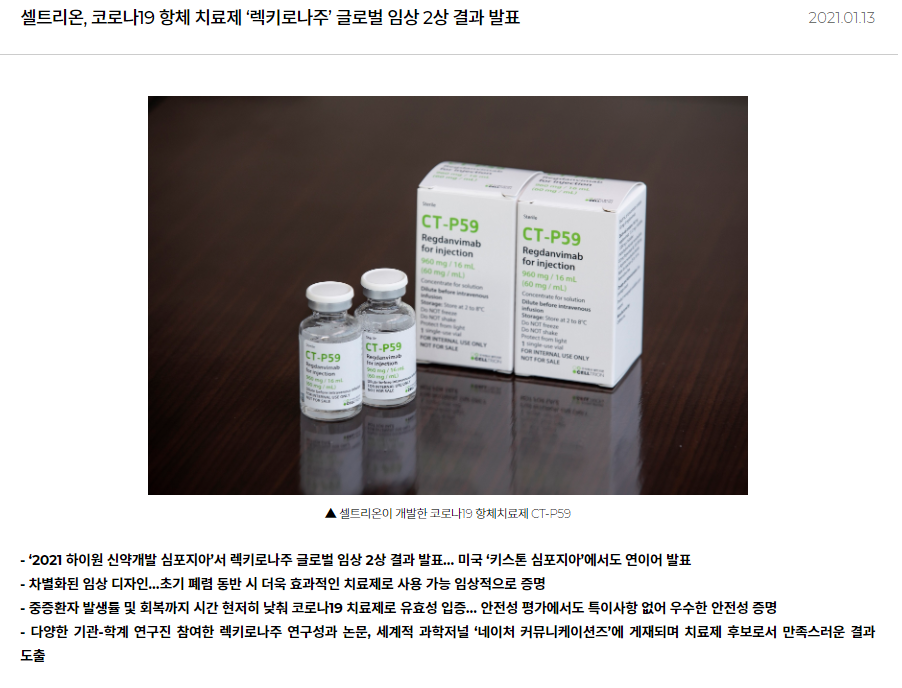
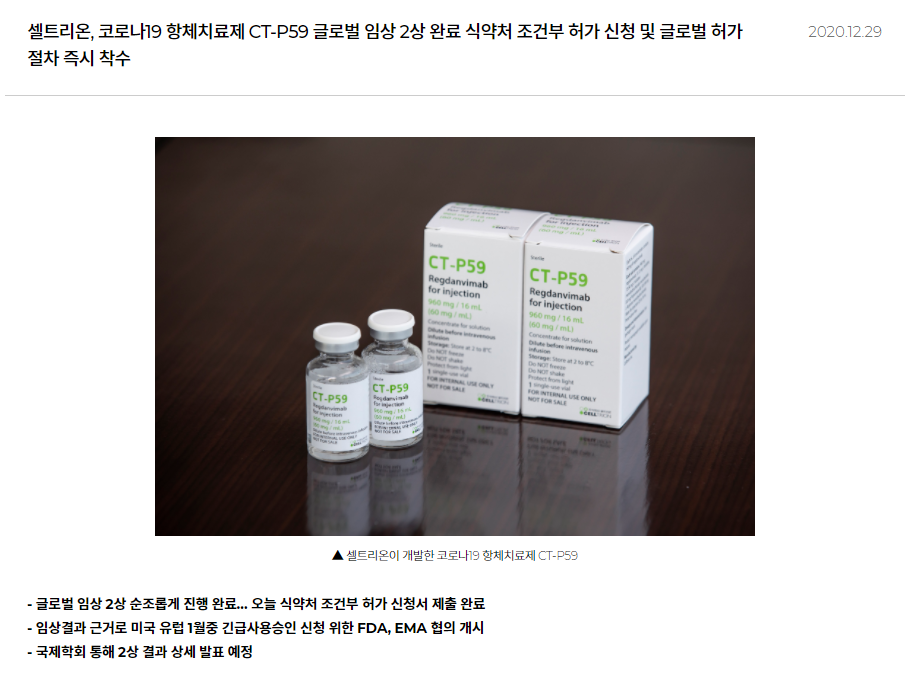
According to a separate statement on December 29, 2020, the company submitted an application for conditional marketing authorisation of the product to the Korean Ministry of Food and Drug Safety.
Copyright © AFP 2017-2026. Any commercial use of this content requires a subscription. Click here to find out more.
Is there content that you would like AFP to fact-check? Get in touch.
Contact us