Hoax advert for Covid-19 vaccine clinical trials circulates across social media platforms in South Korea
- This article is more than five years old.
- Published on November 2, 2020 at 09:00
- 1 min read
- By AFP South Korea, Richard KANG
The image was shared here on a public Facebook group with more than 98,000 members on October 28, 2020.

Part of the Korean-language text in the image translates to English as: “Notice of recruitment for clinical tests of Covid-19 vaccine.
“AstraZeneca is looking for participants for clinical testing… All expenses will be covered, including treatment, check-ups and other medical fees. Each participant will receive 10 million South Korean won (USD 8,850) after trials are completed.
“Please email your application to covid19.trial@astrageneca.com.”
The image carries the logo of AstraZeneca, a British and Swedish pharmaceutical company that also has a branch in South Korea, which operates as AstraZeneca Korea.
The company is currently working with the UK’s University of Oxford to develop a Covid-19 vaccine.
Its AstraZeneca Oxford coronavirus vaccine or AZD1222 vaccine is widely regarded as one of the world's most advanced experimental Covid-19 vaccines in development, with trials underway in the US, Britain, South Africa, Brazil and Japan, according to this AFP report.
The image was also shared here and here on Facebook; here and here on Twitter; here on Naver, alongside similar claims.
However, the claim is false.
AstraZeneca Korea published this statement on October 28, 2020 denying the claim.
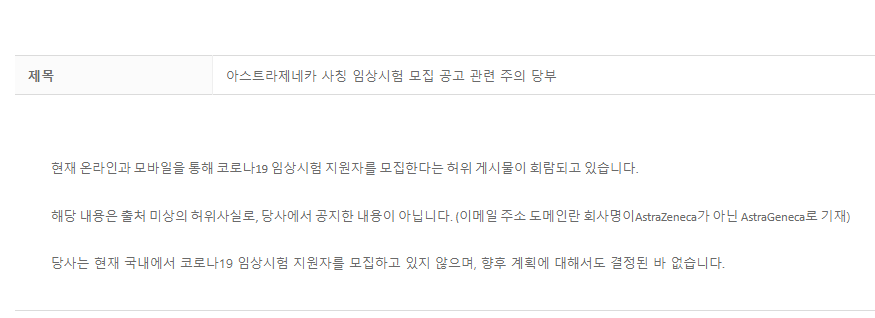
A screenshot of AstraZeneca’s statement published on October 28, 2020
It reads: “There are false posts circulating online and via mobile [messaging] that we are recruiting applications for Covid-19 clinical trials.
“This is not true, and has been distributed by unknown sources. We have not made such an announcement. (The email address in circulation is also incorrect, spelled AstraGeneca, and not AstraZeneca.)
“We are currently not recruiting applicants for Covid-19 clinical trials in Korea, and no decisions have been made on future plans [to do so]”.
In response to the misleading claims, Jeong Gyeong-tae, head of the Vaccine Research Department at South Korea’s National Institute of Health told AFP during a phone conversation on October 29, 2020: “South Korea has not approved any clinical tests for AstraZeneca vaccines in the country.”
Copyright © AFP 2017-2026. Any commercial use of this content requires a subscription. Click here to find out more.
Is there content that you would like AFP to fact-check? Get in touch.
Contact us