'Flawed' paper overstates health risks of abortion pills: experts
- Published on May 5, 2025 at 18:49
- 5 min read
- By Marisha GOLDHAMER, AFP USA
"The Abortion Pill Harms Women: Insurance Data Reveals One in Ten Patients Experience a Serious Adverse Event," says the title of a paper published April 28, 2025 by the Ethics & Public Policy Center (EPPC), a think tank that says its priorities include "pushing back against the extreme progressive agenda while building a consensus for conservatives."

The findings quickly spread across platforms and were held up by conservative media, including Fox News.
Politicians further amplified the claim. Republican Senator Steve Daines of Montana said in a statement: "This recent study is proof that pro-abortion advocates care more about promoting their radical agenda than they do about women's health."
Since the US Supreme Court overturned the federal protection of the right to an abortion in 2022, Texas, Louisiana and other conservative states have adopted tough anti-abortion laws. The court ruled in 2024 that defendants in a case that could have restricted access to mifepristone lacked standing, but the medication remains at the center of efforts to limit abortion (archived here and here).
The EPPC report came days after US Food and Drug Administration (FDA) Commissioner Marty Makary told PBS that while he was not planning to take action on mifepristone, he was looking at new data and would act if it "suggests something or tells us that there's a real signal" (archived here).
But medical abortion has a proven safety record, the ACOG and independent reproductive health experts told AFP.
"Decades of reputable, peer-reviewed, scientific evidence and use data prove that medication abortion is safe and effective," said Stella Dantas, president of ACOG (archived here).
In an April 30 email, she said the EPPC paper is "seriously flawed" and "manipulates data to drive a myth that medication abortion isn't safe."
Medication abortion
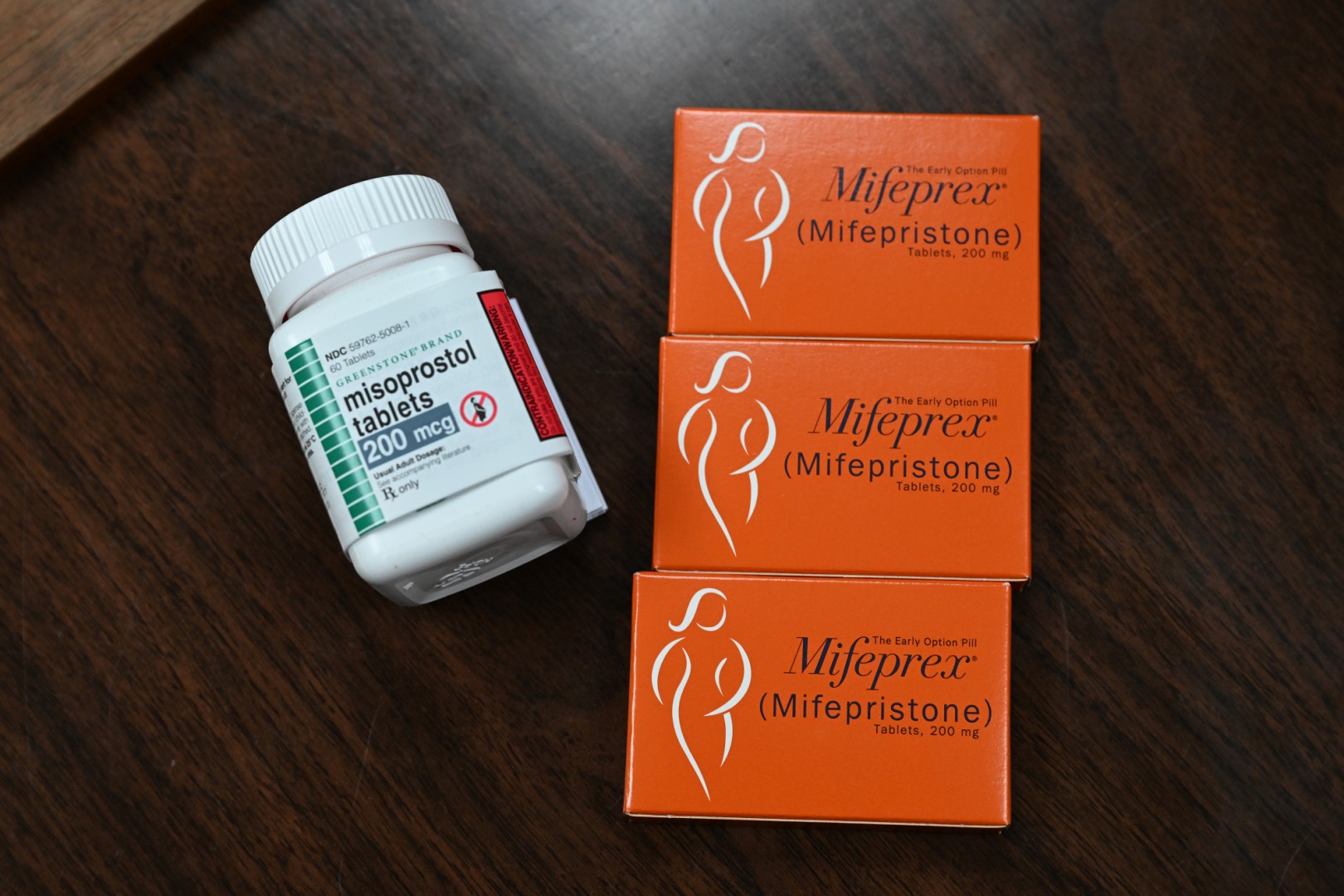
Mifepristone, which prevents pregnancy progression, and misoprostol, which empties the uterus, are approved to terminate a pregnancy up to 70 days of gestation in the United States.
In 2023, they accounted for 63 percent of US abortions.
The FDA says on its website that Mifeprex, the brand name for mifepristone, was first approved in 2000 after a review of the scientific evidence "determined that it was safe and effective for its indicated use" (archived here). The FDA monitors postmarketing data for Mifeprex and its approved generic equivalent, and as of February 2025, it has not identified new safety concerns.
Jack Resneck, former president of the American Medical Association, wrote in a 2024 message supporting access to the medication that mifepristone is "an exceedingly safe and effective prescription drug" (archived here).
Mifeprex maker Danco says on its website that patients will experience cramping and bleeding as part of ending a pregnancy, and as with any prescription drug, there can be rare serious side effects (archived here).
The FDA investigated reports of 36 patient deaths that followed taking mifepristone between September 2000 and December 2024. However, its review of these deaths found: "The adverse events cannot with certainty be causally attributed to mifepristone because of concurrent use of other drugs, other medical or surgical treatments, co-existing medical conditions, and information gaps about patient health status and clinical management of the patient" (archived here and here).
Jennifer Lincoln, a board-certified obstetrics and gynecology physician in Oregon, said postmarketing studies show "thousands of people have used mifepristone in conjunction with misoprostol to have abortions that are without complication 99.7 percent of the time" (archived here and here).
"It is safer to take than Tylenol and far safer than a full-term pregnancy and birth," Lincoln said, referencing the over-the-counter pain medication, which can lead to liver damage if the recommended dosage is ignored (archived here).
Methodology flaws
The EPPC paper is neither peer-reviewed nor published in a medical journal. It was written by the organization's president of public policy and director of data analysis.
The paper's authors say they purchased insurance claim data from 2017 to 2023. From that information, they claim to have identified 865,726 mifepristone abortions by looking for specific procedure, diagnosis and billing codes or prescriptions.
Lincoln pointed to flaws with this strategy, including that it counted "people who got mifepristone and may have taken that alone, without misoprostol -- which is not the evidence-based regimen."
ACOG's Dantas said the methodology could be overestimating the number of people seeking abortion care by including patients who were prescribed mifepristone to deal with complications associated with miscarriages.
Asked about the sample, Hunter Estes, the EPPC's director of communications, dismissed concerns that treatments for a miscarriage would have shown up in the database.
"We would not count that as an abortion, even if she was given mifepristone on that visit," he said in a May 2 email.
After selecting the patients to track, the paper then looked for medical codes for what it categorized as "serious adverse events that occurred within 45 days following the abortion."
Lincoln took issue with the authors including ectopic pregnancy -- a condition where the fertilized egg implants and grows outside of the uterus -- among the negative outcomes.
"Pills can't move your pregnancy out of your uterus and into your fallopian tubes," she said.
Lauren Ralph, associate professor of obstetrics, gynecology and reproductive sciences at the University of California-San Francisco, agreed (archived here).
"Ectopic pregnancy is not caused by a medication abortion, but rather is something that occurs in pregnancy. Abortion medications do not create harm if taken by someone with an ectopic pregnancy," she said in a May 2 email.
The paper also recorded 40,960 visits to a hospital emergency department as serious adverse events following medical abortion.
EPPC's Estes said the report included only visits deemed "likely to be related to the abortion, based on the diagnosis and procedure codes in the insurance records."
But Ralph told AFP a visit to the emergency room alone is not evidence of a serious adverse event.
"Prior research indicates that many people go to the emergency department for follow-up care post-abortion, but this care is often to ensure abortion completion or get reassurance about symptoms rather than for treatment of an adverse event," she said, referencing a 2018 study (archived here).
She said research has consistently found that the rate of serious adverse events is 0.3 to 0.5 percent -- far lower than the figures pushed by EPPC.
When medical abortion was first approved by the FDA in 2000, patients were required to visit a clinic in person to receive the pills (archived here). In December 2021, the policy was updated to allow certain providers to prescribe the medication via telehealth appointments (archived here). Further policy changes allowed pharmacies to be certified to dispense mifepristone directly to patients, and in March 2024, major US chains CVS and Walgreens announced plans to fill prescriptions in Massachusetts and other states where the medication is legal.
The EPPC called on the FDA to "reinstate its earlier, stronger patient safety protocols." But a peer-reviewed study published in Nature Medicine in February 2024 found: "Telehealth medication abortion is effective, safe and comparable to published rates of in-person medication abortion care" (archived here).
Read more of AFP's reporting on misinformation about abortion here.
Copyright © AFP 2017-2026. Any commercial use of this content requires a subscription. Click here to find out more.
Is there content that you would like AFP to fact-check? Get in touch.
Contact us