FDA's updated Covid vaccine guidance revives false claims
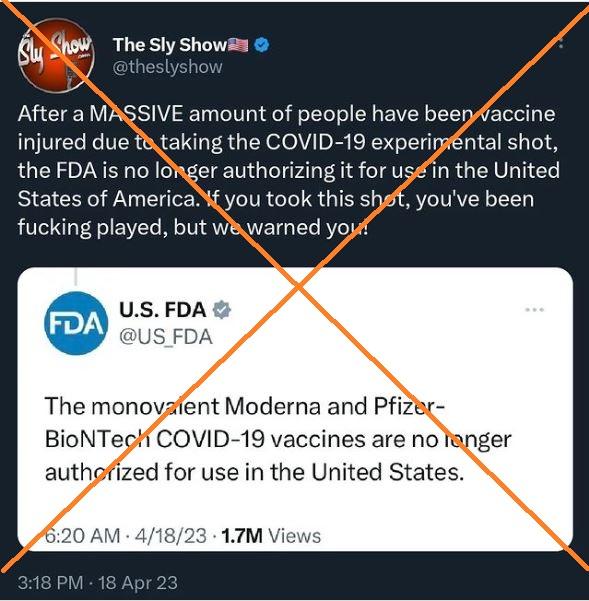
"After a MASSIVE amount of people have been vaccine injured due to taking the COVID-19 experimental shot, the FDA is no longer authorizing it for use in the United States of America," says an April 18, 2023 tweet also shared on Instagram. "If you took this shot, you've been fucking played, but we warned you!"
Another Twitter user said: "Today the FDA quietly banned the covid vaccines... This is their way is admitting they fucked up with out actually saying they fucked up."
Others shared a similar message. The claims follow a wave of Covid-19 vaccine misinformation on social media, much of which has been debunked by AFP and others.
The FDA on April 18 updated its Covid-19 vaccine recommendations, saying the monovalent shots from Pfizer-BioNTech and Moderna are "no longer authorized for use in the United States."
But the agency has said the jabs were not banned -- and that the changes reflect the evolution of the coronavirus, not safety concerns.
"It's probably worth clarifying that the monovalent vaccines are still approved (licensed). That hasn't changed," the FDA said in an April 18 tweet responding to a question from Twitter owner Elon Musk. "But they are no longer authorized for *emergency use* in the United States."
Today, FDA amended the EUAs of the Pfizer-BioNTech and Moderna COVID-19 bivalent mRNA vaccines to simplify the vaccination schedule for most individuals. https://t.co/Cd74KB3n9p
— U.S. FDA (@US_FDA) April 18, 2023
What you need to know. pic.twitter.com/9394LP1HvR
Full FDA approval requires more data and longer monitoring of clinical trial participants than emergency use authorization.
The agency granted the latter to monovalent Covid-19 vaccines in late 2020, while full approval came in August 2021. The FDA authorized bivalent booster doses for emergency use in August 2022.
Peter Marks, head of the FDA Center for Biologics Evaluation and Research, told media April 18 that the original vaccines would retain their biologics license applications and would likely be used for future updates to the shots.
The FDA said the new guidance aims to simplify the vaccine schedule for most people, including unvaccinated adults. The agency now recommends a single dose of the bivalent vaccine -- which includes a component of the omicron variant -- rather than multiple doses of the monovalent shot.
"Evidence is now available that most of the US population 5 years of age and older has antibodies to SARS-CoV-2, the virus that causes Covid-19, either from vaccination or infection that can serve as a foundation for the protection provided by the bivalent vaccines," Marks said in an April 18 press release (archived here).
Andrew Pekosz, vice chair of microbiology and immunology at Johns Hopkins University, agreed that the guidance reflects the state of the virus and is not related to safety issues.
"Numerous studies in people have shown that the safety profiles of both the monovalent and bivalent Covid-19 vaccine formulations are nearly identical," Pekosz said in an April 19 email. "This was expected because both use the same mRNA vaccine platform, (they) just have different versions of the spike protein in them."
He added that the bivalent booster "recognizes omicron variants better, so that makes it a better choice right now given that only omicron variants are circulating."
The US Centers for Disease Control and Prevention and other public health authorities say Covid-19 vaccines are safe and effective at preventing severe illness and death. There are a few rare side effects, but studies indicate most of them are mild and temporary.
AFP has fact-checked other false and misleading claims about vaccines here.
Copyright © AFP 2017-2026. Any commercial use of this content requires a subscription. Click here to find out more.
Is there content that you would like AFP to fact-check? Get in touch.
Contact us