South Korean officials set to investigate nasal dilator over unproven Covid-19 prevention claim
- This article is more than five years old.
- Published on January 8, 2021 at 08:45
- 2 min read
- By Richard KANG, AFP South Korea
The misleading claim about the product, named “Kovagi”, was shared here on Facebook on December 21, 2020.
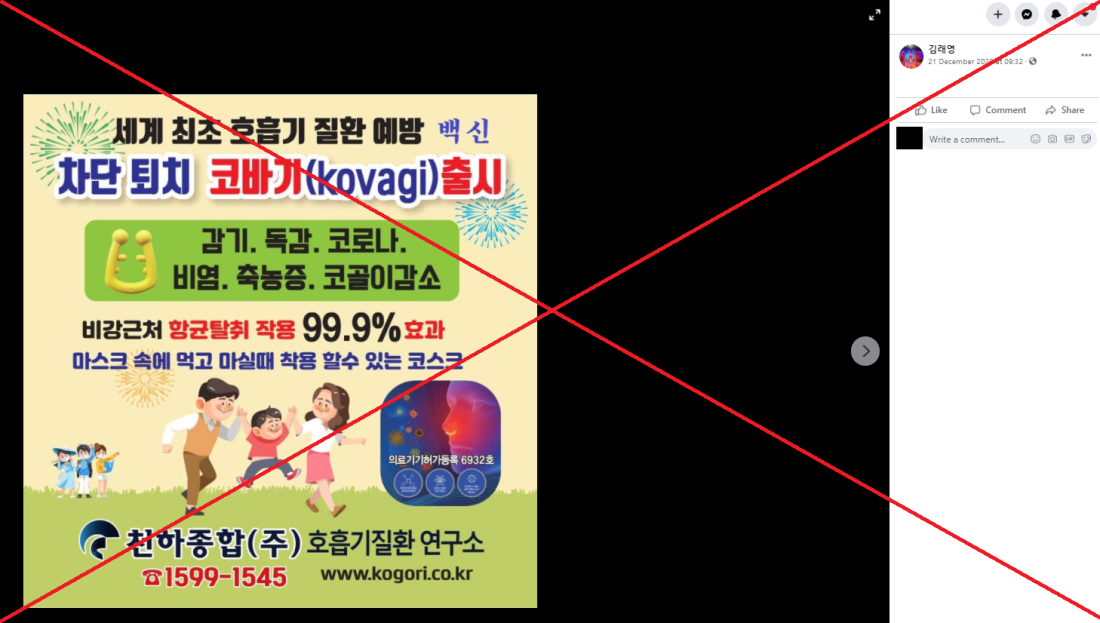
Part of Korean-language text superimposed on the image translates to English as: “The world's first vaccine that prevents respiratory disease.
“Prevent and kill. Kovagi launched.
“Common cold, flu, Covid-19, rhinitis, sinusitis and reduction of snoring.
“99% antibacterial and deodorisation effectiveness around the nasal area.
“It's a 'nosesk' [nose+mask] that you can wear while eating or drinking.”
A similar claim has been shared on Facebook here, here and here; and on Korean blog site Naver here.
The claim is misleading.
Kim Hyun-sun, head of Cyber Investigation Team at South Korea's Ministry of Food and Drug Safety, told AFP it would launch an investigation into the product as the claim in the social media posts was “likely to be false”.
“The product is registered as a nasal dilator; the advertisement is likely to be false, and its claim [about Covid-19] is also likely to be false,” she said during a phone interview on January 6, 2021. “We will launch an investigation on both manufacturer and the product to check their claim, but it will take some time.”
The information on the ministry’s website shows that the product is registered as a medical device and designed to dilate the nasal valves.
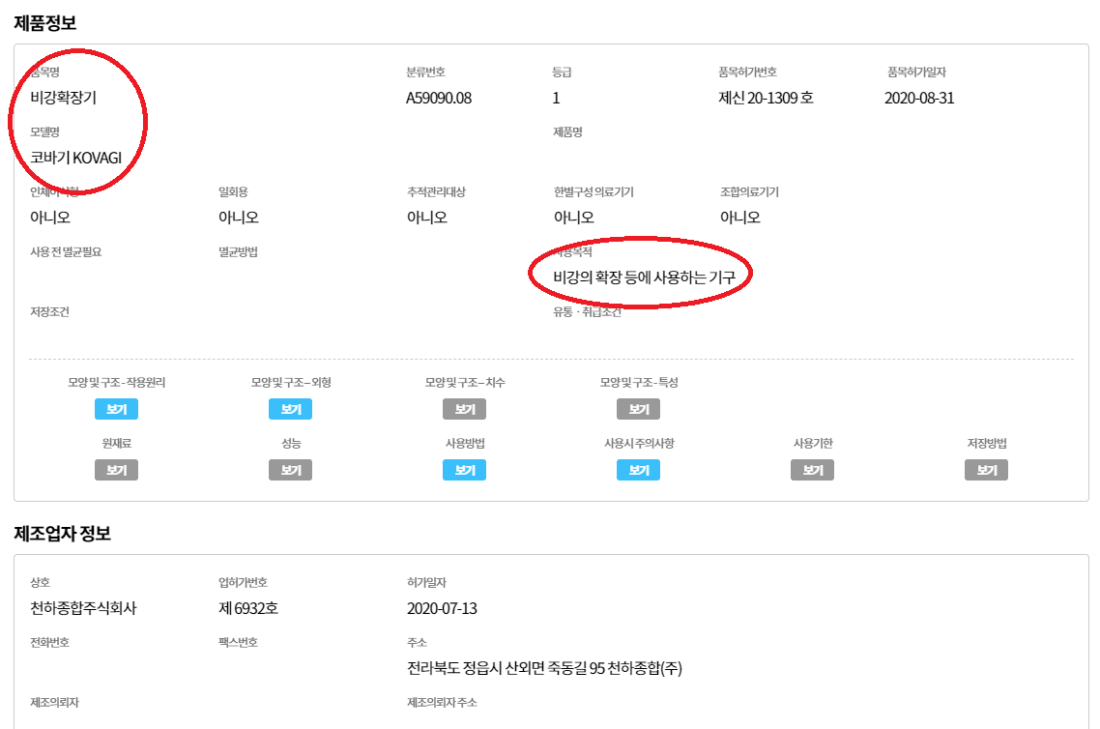
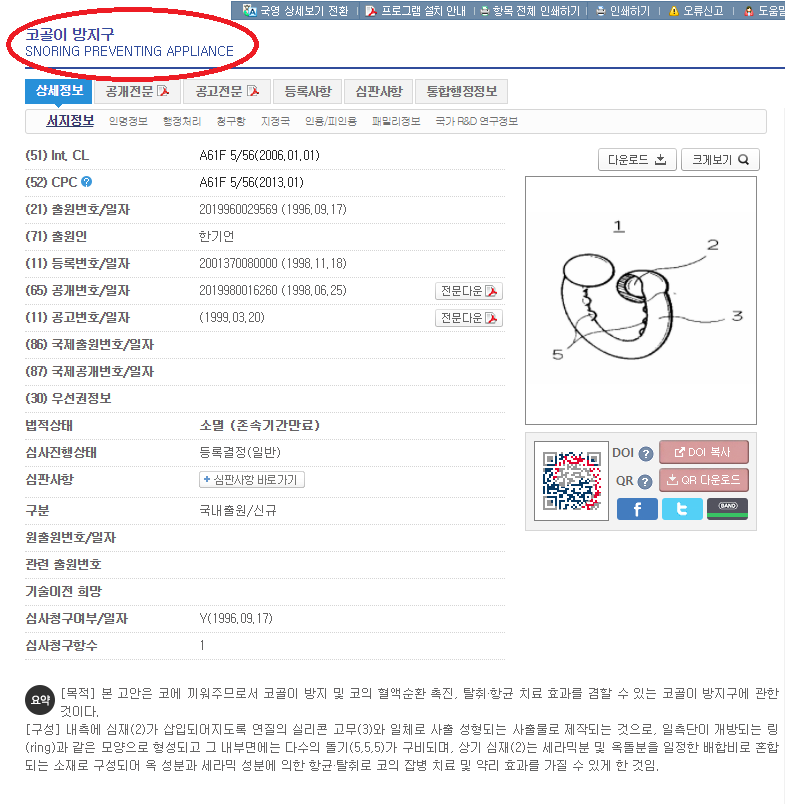
The product has acquired a patent from the Korean Intellectual Property Office as a “Snoring Preventing Appliance.”
Below is a screenshot of the device’s patent on the Korean Intellectual Property Office’s website:
Kim Byung-yong, a researcher at the Korea Far Infrared Association (KFIA), told AFP that the product had been tested for its effectiveness in protecting the user from bacteria, rather than viruses.
“It is true that KFIA issued a certificate to the manufacturer of the product in February 2019 to confirm its antibacterial effectiveness, but the test was to see its effectiveness against colon bacillus and bacillus pyocyaneus,” he said in a phone interview on January 6, 2021. “Bacteria and viruses are different. Proven antibacterial effectiveness does not mean it’s also effective in preventing Covid-19.”
No evidence that the product is effective in preventing Covid-19 infection is shown on the product’s official website here.
Copyright © AFP 2017-2026. Any commercial use of this content requires a subscription. Click here to find out more.
Is there content that you would like AFP to fact-check? Get in touch.
Contact us