
Malaysia govt warns against cosmetic product found containing controlled substances
- This article is more than one year old.
- Published on October 15, 2024 at 06:11
- Updated on October 16, 2024 at 06:17
- 2 min read
- By Najmi Mamat, AFP Malaysia
The product -- called Dnars Gold Debalen Cream -- was falsely advertised as "free from prohibited substances" in a Malay-language Facebook post shared on September 29, 2024.
It claimed the face cream helps "remove freckles, acne and scars" and treats "pigmentation and dark spots".
Several images attached to the post show the cream packaging that comes in a set of different boxes.
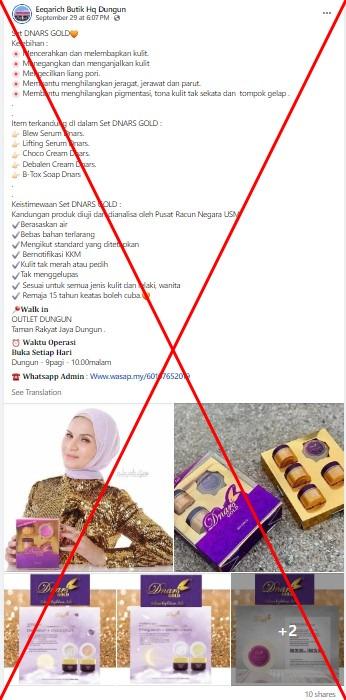
Similar posts advertising the cream were shared elsewhere on Facebook here, here and here.
Malaysia's health ministry, however, warned the cream contains potentially harmful controlled medications.
Recalled product
Malaysia's Ministry of Health announced on September 30 that it had found Dnars Gold Debalen Cream contains hydroquinone, tretinoin and betamethasone 17-valerate -- substances that cannot be used without a prescription (archived link).
The ministry warned the public that unsupervised use can cause side effects such as "redness of the skin, cause the skin to become thin and prone to irritation as well as increase the risk of being absorbed into the blood circulation system."
It ordered the immediate halt of all sales of the affected products, and for sellers to contact the nearest district health office so remaining stocks could be seized.
The sale and distribution of the products violate the country's Control of Drugs and Cosmetics Regulations 1984, which punishes offending individuals and firms with fines and jail time, the ministry added.
The ministry also said the products are "no longer allowed to be sold in Malaysia."
Below is a screenshot comparison between the product shown in the false post (left) and example images of Dnars Gold Debalen Cream included in the ministry's advisory (right):

Side effects
According to the Malaysian Health Ministry, consumers must ask a health professional for advice first before using products that contain hydroquinone, clindamycin, metronidazole, tretinoin and betamethasone 17-valerate (archived link).
While some of these substances help improve skin conditions, unsupervised use could lead to harmful side effects, Dr Felix Yap Boon Bin, a consultant dermatologist at Sunway Medical Centre in Malaysia, told AFP on October 9 (archived link).
"High concentration of hydroquinone leads to bleaching of the skin called leukoderma leading to pale white skin on exposed areas. The permitted concentration in Malaysia is only up to 4 per cent."
"Betamethasone and tretinoin can only be used by experienced medical practitioners with limited duration of use."
In Malaysia, the list of qualified dermatologists can be obtained from the Dermatological Society of Malaysia website (archived link).
AFP has previously fact-checked misinformation related to consumer safety in Malaysia.
Corrected the name of the law mentioned in tenth line to "Control of Drugs and Cosmetics Regulations 1984"October 16, 2024 Corrected the name of the law mentioned in tenth line to "Control of Drugs and Cosmetics Regulations 1984"
Copyright © AFP 2017-2026. Any commercial use of this content requires a subscription. Click here to find out more.
Is there content that you would like AFP to fact-check? Get in touch.
Contact us
