
Online ads push unproven 'lung cleansing' products
- This article is more than one year old.
- Published on August 21, 2024 at 21:56
- Updated on August 23, 2024 at 15:25
- 4 min read
- By Daniel Patrick GALGANO, AFP USA
"Unbelievable! A Revolutionary Drug-Free Remedy That Will Cleanse Your Lungs & Help You Breathe Easier," says an August 13, 2024 post on Facebook with thousands of interactions.
A video accompanying the post claims that the product will "heal" and "rejuvenate" users' lungs and remove waste from the respiratory tract, which it says can "reduce the risk" of lung cancer.
The same products are also advertised alongside similar claims elsewhere on Facebook, X, and TikTok -- including in Spanish -- and are sold by online retailers via Amazon and eBay.
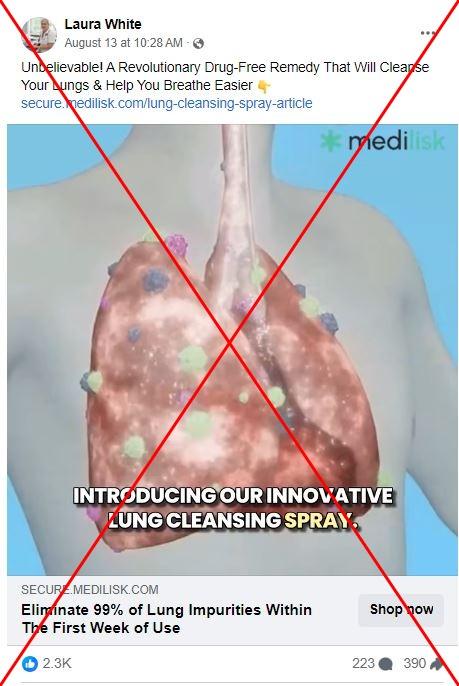
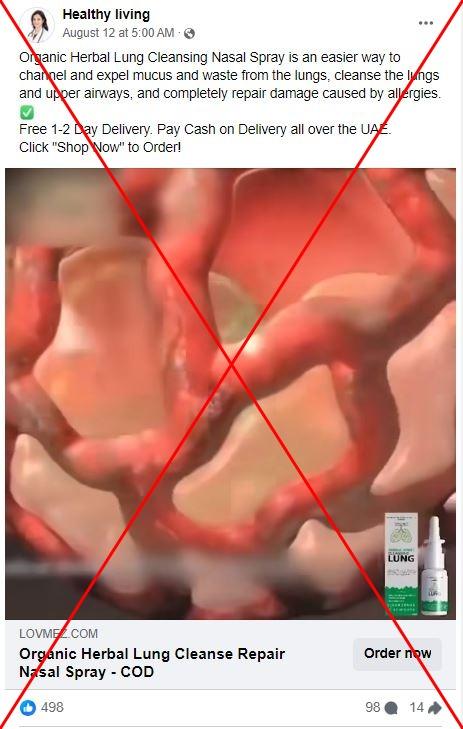
Many of the posts promote a product from a company called Medilisk, which claims its lung spray can "liquify mucus" and can help treat respiratory diseases including asthma, emphysema, chronic obstructive pulmonary disease (COPD), bronchiectasis and chronic bronchitis. Some suggest it can help people "recover" from the damage caused by smoking.
But Medilisk currently holds an "F" rating with the Better Business Bureau. The nonprofit has published dozens of public complaints about the company (archived here).
The Medilisk website contains a disclaimer admitting that its products have not been reviewed by the US Food and Drug Administration (FDA). However, a similar product that uses much of the same imagery as Medilisk is being promoted on Facebook by a Hong Kong-based company under the brand "LOVILDS" with claims that the product is FDA-approved.
A search of the FDA's approved drugs database shows the agency has not approved the over-the-counter spray or the ingredients on the product labels for treating respiratory illnesses.
Erica Salem, senior director of strategy, programs and policy at the Respiratory Health Association, a Chicago-based nonprofit, said there is currently "no scientific evidence" behind claims that lung spray products can remove toxins from a person's lungs. She said people suffering from asthma or COPD should seek advice from their doctor before starting any time of treatment.
"Avoid putting things into your lungs, including products that aren't FDA approved and recommended by your clinician," she said in an interview with AFP on August 20, 2024.
Salem said although the product lists natural herbs and oils including peppermint and eucalyptus as its main ingredients, health professionals "don't know" what effect they could have on lung health.
"If you want to reduce the risk of lung cancer, avoid smoking, stay away from secondhand smoke," she said.
Suspicious endorsement
According to the Meta Ad Library, which tracks advertisements on the company's social media platforms, one account promoting the spray has launched almost 80 advertisements on Facebook, Instagram, and Messenger since May 2024, targeting former smokers and people with respiratory conditions, with various other profiles promoting dozens of others.
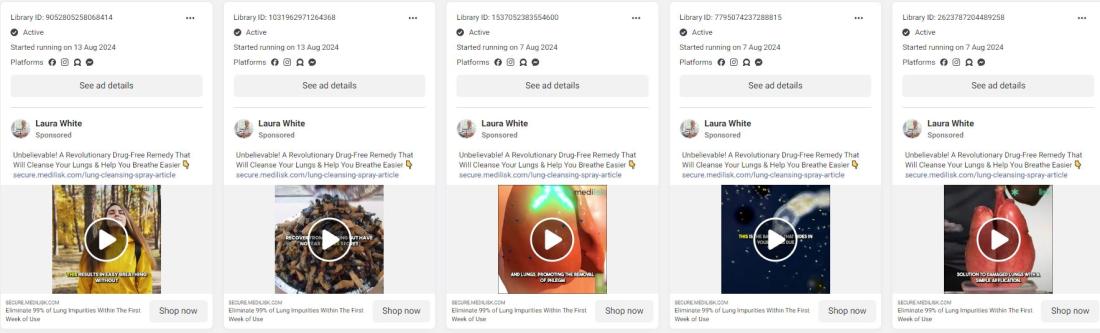
The ads and the Medilisk website feature a purported endorsement from "Dr Emma Foster" or a photo of the same woman dressed in a lab coat using the name Laura White.
No medical doctors with either of those names are currently licensed in the US states of Florida or New York, where Medilisk and its parent company are headquartered, according to the respective states' medical boards (archived here).
A reverse image search of reveals the person in the photo is actually Ludmila Bezdíčková, a doctor working in the Czech Republic (archived here).
Contacted by AFP, Bezdíčková said the picture used on the Medilisk website is an "altered photo and identity fraud." She said she could sue the company for misusing her image.
'No convincing evidence'
Tianshi David Wu, an assistant professor of pulmonary and critical care at the Baylor College of Medicine in Houston, Texas (archived here), recommended that patients avoid unproven and unregulated lung "cleansing" sprays like those promoted on Facebook.
"The lungs already have an elaborate system to protect against infections and remove inhaled irritants. In general, there is no convincing evidence to support these products' claims of disease prevention," he said in an email sent to AFP on August 15, 2024.
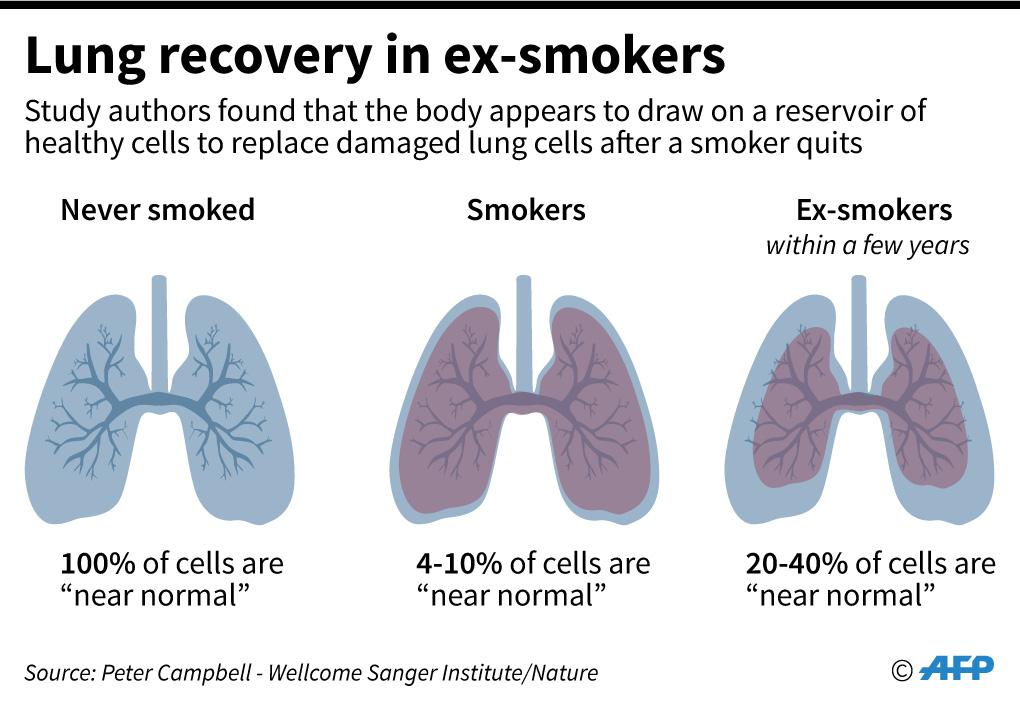
According to the Lung Health Foundation, a Toronto-based charity: "These types of products are considered alternative therapies and are not evidence-based, regulated, or recommended. The therapeutic claims from the use of their products can be misleading, fearmongering and are often unsubstantiated without strong scientific backing."
It recommended patients discuss any new products they are considering with a healthcare provider -- advice echoed by the American Lung Association.
AFP contacted Medilisk for comment, but no response was forthcoming.
AFP has previously debunked additional posts promoting unsubstantiated respiratory health products.
Ladka Mortkowitz contributed reporting for this fact-check.This story was updated to add comment from Ludmila Bezdíčková.
August 23, 2024 This story was updated to add comment from Ludmila Bezdíčková.
Copyright © AFP 2017-2026. Any commercial use of this content requires a subscription. Click here to find out more.
Is there content that you would like AFP to fact-check? Get in touch.
Contact us
