
Philippine FDA boss targeted with edited pictures promoting unregistered eye 'treatment'
- This article is more than one year old.
- Published on February 29, 2024 at 07:17
- Updated on February 29, 2024 at 07:19
- 3 min read
- By Jan Cuyco, AFP Philippines
One of the edited photos shows Zacate, the Philippine FDA's director-general, giving a presentation next to posters that say "Introducing the product Eyes Blue" and "Eyes Blue: Protect your own eyes".
The picture was shared on Facebook on December 20, 2023 in a Tagalog-language post that claimed the agency held a "workshop for strategic cooperation" and a "quality inspection" of the touted product in the Philippine capital Manila.
The post includes hashtags that say "treatment" and mention eye conditions such as cataract, glaucoma and macular degeneration.
Similar pictures surfaced on Facebook on January 5, 2024. They appear to show Zacate speaking at a different event alongside posters that promote the same pill.
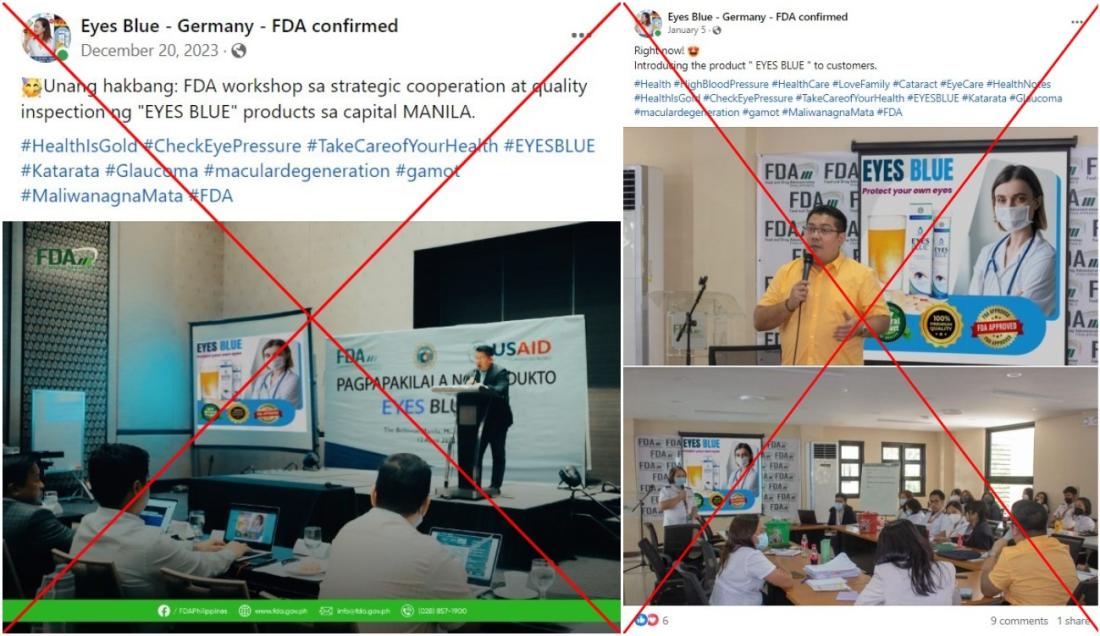
The edited pictures circulated on similar Facebook pages here and here, and on the product's website selling the efferverscent tablets.
Zacate told AFP the posts were "fake". He said he did not endorse the product and that he was in fact prohibited from promoting any medical products due to his job at the FDA.
Manipulated images
A reverse image search on Google found the original version of the first picture in a post on the FDA's official Facebook page from April 13, 2023 (archived link).
In the original picture, the poster behind Zacate says: "Dialogue on Strengthening the Medical Device Regulation in the Philippines" -- not "Introducing the product Eyes Blue".
The poster to Zacate's right shows a photo of him -- not text reading "Eyes Blue: Protect your own eyes".
According to the Facebook post, the photo was taken at an FDA meeting with the United States Agency for International Development on beefing up the regulation of medical devices in the Philippines.
Below is a screenshot comparison of the altered image (left) and the original photo (right), with differences highlighted.

Google keyword searches found the other pictures on the Philippine FDA's website (archived link).
In the original picture, the projector screen behind Zacate is blank. It does not show an advertisement for "Eyes Blue".
The FDA posted the same photos on its Facebook page on February 8, 2023. It said they were taken at an open forum with Zacate and staff from the Center for Drug Regulation and Research, an FDA department, to discuss workplace concerns. (archived link).
Below is a screenshot comparison of the altered images (left) and the original photos (right), with differences highlighted.

There is no trace of "Eyes Blue" on the FDA's databases for registered drug and food products, as of February 27, 2024 (archived links here and here).
Doctors' warning
Moreover, doctors warned against taking unregistered treatments for eye conditions.
Alex Pisig, a retina specialist at the Asian Eye Institute in the Philippines, previously told AFP that cataracts can only be treated by surgery.
Lifestyle changes such as quitting smoking can also slow down the formation of cataracts, which happens when the natural lens of the eyes becomes cloudy, he added.
Glenn Carandang, an eye surgeon at St. Luke's Medical Center in Manila, said prescribed medication is required to treat glaucoma, a disease that damages eye nerves.
Both doctors said treatment of age-related macular degeneration disease varies from injection of drugs into the eye and laser therapy to mineral supplements for patients with milder disease.
The American Academy of Ophthalmology warned against buying "over-the-counter products that are advertised to save your sight" (archived link).
AFP has previously debunked Facebook pages sharing manipulated images to market unregistered medical products, including for arthritis, thyroid problems and high blood pressure.
Copyright © AFP 2017-2026. Any commercial use of this content requires a subscription. Click here to find out more.
Is there content that you would like AFP to fact-check? Get in touch.
Contact us
